The Road to Solar Fuels Hits a Speed Bump
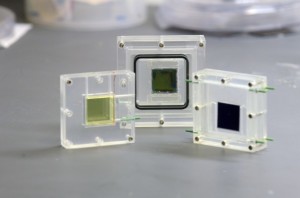
When I visited Lawrence Berkeley National Laboratory in March, Frances Houle, the deputy director of the Joint Center for Artificial Photosynthesis, showed off one of the center’s latest advances. It is a device that breaks down water into hydrogen and oxygen in sunlight. The lab’s researchers had previously used artificial light to drive the process; this was the first time they were doing it with natural light. Fixed to a thin metal stand on the roof of the center’s building above Berkeley, with a spectacular view west across San Francisco Bay, the small device has a solar cell that supplies the energy needed for a chemical catalyst to split the water. At the top of the device, pure hydrogen bubbled up.
Created in 2010 under Secretary of Energy Steven Chu, the center, commonly called JCAP, has an audacious goal: to create fuels using only sunlight, carbon dioxide, and water (see “Artificial Photosynthesis Effort Takes Root”). Done economically, that would be a Promethean achievement, representing a huge step toward solving the two outstanding challenges in shifting from fossil fuels to renewable energy: storing large amounts of energy for later use, and powering forms of transportation that cannot easily run on batteries.
“All of the studies of a clean energy system I’ve ever seen identify the same two technology gaps,” says Nate Lewis, the center’s founding director. “Massive grid-scale energy storage to compensate for the intermittency of wind and solar power, and an energy-dense, carbon-neutral liquid transportation fuel.” Turning sunlight into fuel would enable solar energy captured during the day to be stored, transported, and used when the sun’s not shining. The same fuel could replace fossil fuels that power today’s aircraft and ships. “There are no such things as a plug-in electric airplane or ship,” adds Lewis.
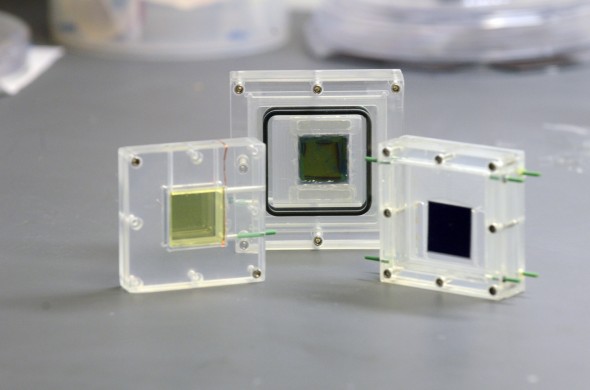 One of JCAP’s primary achievements has been solar-energy-driven devices that can split water into hydrogen and oxygen—an important first step on the road to artificial photosynthesis.
One of JCAP’s primary achievements has been solar-energy-driven devices that can split water into hydrogen and oxygen—an important first step on the road to artificial photosynthesis.
Hosted at Caltech and Lawrence Berkeley Lab, JCAP was originally funded with $122 million over five years, and its funding was renewed (albeit at a lower level) last year. Now headed by Harry Atwater, a Caltech professor of applied physics, it has made some impressive achievements in its six years of existence. Most notably, JCAP scientists have succeeded in building devices like the one I saw, prototypes that can split water into hydrogen and oxygen at 10 times the efficiency of photosynthesis. That is an important first step to artificial photosynthesis; the next step would be combining hydrogen with carbon dioxide to produce “solar fuels” that could replace fossil fuels.
In the last year, though, JCAP has made a significant shift in direction. The Department of Energy renewed its funding last year at $15 million a year, nearly 40 percent below the rate for the previous five-year period. What’s more, DOE officials instructed the scientists to refocus their efforts away from making devices that could be commercialized in the next several years and toward basic scientific research on the complex processes underlying artificial photosynthesis. JCAP’s original goal, according to the 2010 announcement of its founding, was “to develop an integrated solar energy-to-chemical fuel conversion system and move this system from the bench-top discovery phase to a scale where it can be commercialized.” Now its mandate no longer goes beyond the discovery phase.
“In its first five years, JCAP focused primarily on hydrogen fuel,” says Christopher Fecko, the program manager for JCAP in the DOE’s Office of Science. Tackling the underlying scientific challenge of full artificial photosynthesis will require “basic, transformational scientific research and discovery that will eventually enable these technologies,” he says, “and we are making excellent progress. Technology deployment and commercial production are in the future.”
“In the future” could mean in five years or a few decades. The retreat from hammering out a working solution as fast as possible is an admission that JCAP’s original goal of artificial photosynthesis is a lot harder and further off than scientists understood in 2010. It’s also a tactical decision not to spend federal money creating a working device to produce hydrogen fuel, even though that technology is much closer to commercialization.
“My original vision was to give the scientists a lot of leeway and let them choose the direction they think is most promising so they can actually put something together,” says Chu, who left the DOE in 2013 and is now a professor of physics and molecular and cellular physiology at Stanford. Today, he adds, “I don’t think they are being given the freedom I was envisioning.”
The first step in photosynthesis is to split water into hydrogen and oxygen, which is released as a by-product. The hydrogen then reacts with carbon dioxide to produce carbohydrates, which fuel plants’ growth. Artificial photosynthesis seeks to use the same inputs—solar energy, water, and carbon dioxide—to produce energy-dense liquid fuels. If those fuels were derived from carbon dioxide captured from air, the process could be carbon-neutral—in other words, it would add no new emissions of greenhouse gas to the atmosphere.
 Based at Lawrence Berkeley National Laboratory, JCAP was conceived as a Bell Labs–like incubator of technologies that could be quickly commercialized.
Based at Lawrence Berkeley National Laboratory, JCAP was conceived as a Bell Labs–like incubator of technologies that could be quickly commercialized.
Since 2010, when JCAP was formed, research on artificial photosynthesis and solar fuels has gained momentum worldwide. There are national research and development consortia attacking the problem in Japan, Sweden, and other countries. The DOE supports other programs, such as the Energy Frontier Research Center for Solar Fuels at the University of North Carolina. Startups like Opus12, founded by a trio of Stanford grad students, and Liquid Light, headed by Princeton chemistry professor Andrew Bocarsly, are trying to build businesses out of artificial photosynthesis.
The question is, will they bear fruit in time to help limit global climate change? One problem is that making solar fuels carbon-neutral will require entire new technologies and infrastructures to capture carbon from the air or emissions from fossil-fuel plants.
The other problem is that converting carbon dioxide to complete the photosynthesis process is very, very hard. It involves six separate chemical steps, and there is no known catalyst that will convert carbon dioxide into fuel efficiently and selectively, the way there is for the water-splitting reaction.
“The challenge that JCAP is tackling in its second five years is actually more fundamental and difficult in many respects than the hydrogen-fuel effort, but of great potential payoff,” says Fecko.
The shift, says Harry Atwater, Lewis’s successor as JCAP director, “has driven the entire enterprise not to prototyping and scale-up activities, but to a much stronger focus on basic research.” That focus aligns with the goals of the DOE’s Basic Energy Sciences program, which controls JCAP’s funding. But it won’t get us any closer, in the near term, to actually building devices and creating an industry around solar fuels.
 JCAP scientists Sonjia Francis (right) and Dan Torelli (left) investigate the electrochemical reduction of carbon dioxide into liquid fuel. JCAP has shifted its focus away from hammering out solutions and toward basic scientific research.
JCAP scientists Sonjia Francis (right) and Dan Torelli (left) investigate the electrochemical reduction of carbon dioxide into liquid fuel. JCAP has shifted its focus away from hammering out solutions and toward basic scientific research.
That’s why Nate Lewis believes the shift away from prototyping and scaling up hydrogen-generating devices was a mistake. Hydrogen itself is a useful end product, Lewis argues. It can be burned directly in modified internal-combustion engines. It can be converted to synthetic fuel via the Fischer-Tropsch process. It can be used in fuel cells to store energy and to produce electricity, leaving only water as a waste product. The water-splitting prototypes developed at JCAP will still require extensive development to be turned into useful commercial devices. But Lewis is confident he can get there, and in a relatively short time: “If we had less than $5 million a year, I’m pretty confident that we could get there in five years, and that’s the first time I’ve said that,” he says.
For now, however, that work will not be carried out through JCAP. Lewis supports continued research into full-scale artificial photosynthesis. But he views JCAP’s redirection as effectively abandoning a promising clean-energy technology—water splitting for hydrogen production—that could be commercialized much sooner. Basic research on carbon dioxide conversion “is a very narrowly defined scope and focus,” he says. “Solar fuels should be much broader than picking one specific way to get there and defunding other options.”
Atwater and Fecko say the invention of a water-splitting device was an important milestone that other labs and other researchers can bring to fruition. “Our job is to make research advances that give rise to technology options,” says Atwater. “We’re scientists—we can’t push the ball all the way to the goal line.”
Reaching the goal line, though, was the original aim set out by Chu when the center was founded. In a sense, JCAP is a case study in the promise and the perils of long-term federal funding for energy technologies. Chu speaks ruefully of the road not taken by the program he created in 2010. Basic science is a necessary and wonderful thing, he says. “But it’s not what I had in mind.”

Leave a Reply